Active and Stable Plasma-Enhanced ALD Pt@Ni-YSZ Hydrogen Electrode for Steam Reversible Solid Oxide Cells
Abstract
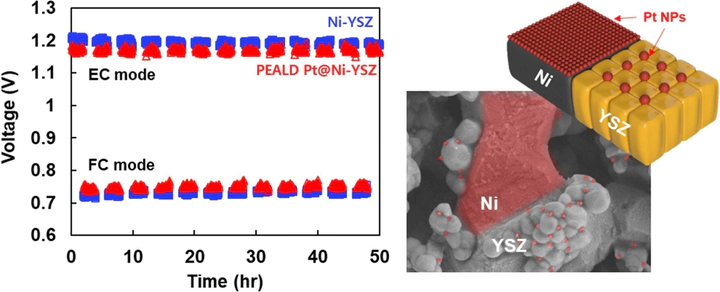
Designing and fabricating active and thermally stable bifunctional catalysts with minimal noble metal loadings are crucial for reversible solid oxide cells (rSOCs). This study employed Pt nanoparticles fabricated via plasma-enhanced atomic layer deposition (PEALD) to a nickel-yttria stabilized zirconia (Ni-YSZ) electrode to serve as effective catalysts in fuel cell and electrolysis modes. Despite the minimal Pt catalyst loading (<1 μg cm–2), the PEALD Pt@Ni-YSZ catalytic electrode exhibited superior electrochemical performance, (20 % higher than the bare cell), both in the fuel cell and electrolysis modes. Remarkably, this performance is sustained without any degradation over a 50 h duration at 700 °C. Dissimilar stabilization behaviors of the Pt catalysts occurred as distributed fine particles on Ni and anchored coarsened particles at the grain boundaries on YSZ surfaces. Furthermore, the mechanism of the enhanced hydrogen evolution/oxidation reactions with the PEALD Pt@Ni-YSZ electrode was verified using density functional theory simulations.