Abstract
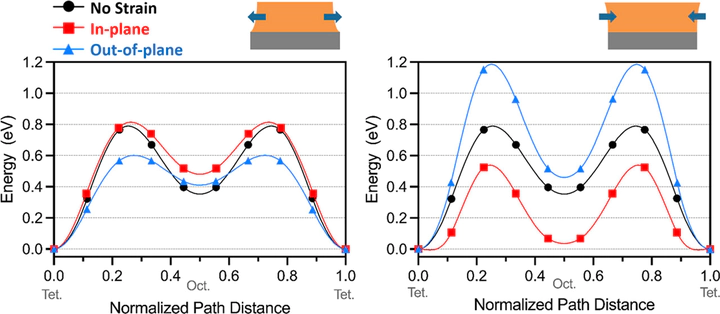
Successful deployment of a Mg-ion battery requires cathodes that can achieve reversible Mg intercalation and high energy density. Recent theoretical and experimental studies indicated that the overall transport is likely limited by sluggish Mg transport at the cathode–electrolyte interface and not Mg diffusion through bulk. In this work, we investigated the surface electrochemical activity of Mg ions by using a spinel-structured manganese oxide thin-film model system and in situ X-ray scattering. In combination with post-mortem microscopy analysis, we found that magnesium insertion was more favorable than subsequent extraction near the surface of the MgxMn2O4 film, resulting in overmagnesiation, and eventually amorphization of the surface. This structural irreversibility and high overpotential required for Mg extraction could explain significant voltage hysteresis and Mg surface enrichment previously observed in bulk cathodes. Density functional theory calculations suggested that the tendency for the Mg surface enrichment could be associated with Mg diffusion kinetics, which varies with the strain state evolved due to constrained film volume change during Mg insertion and extraction. Particularly, out-of-plane Mg migration was predicted to be favorable in the tensile strain rather than in the compressive case.