Investigation of Rechargeable Calcium Metal-Selenium Batteries Enabled by Borate-Based Electrolytes
Abstract
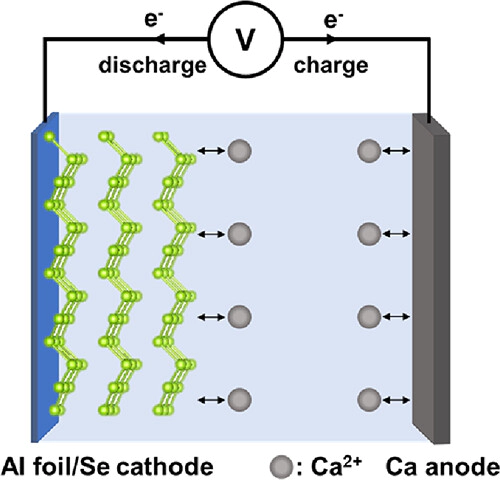
Calcium-ion batteries (CIBs) are a promising next-generation energy storage system given the low redox potential of calcium metal and high abundance of calcium compounds. For continued CIB development, the discovery of high energy density calcium ion cathodes is needed to achieve practical energy density values. Here, we report on the use of elemental Se as a promising candidate for a high-capacity cathode material for CIBs that operates via a conversion mechanism in a Ca metal battery at room temperature. The Se electrodes demonstrate a reversible specific capacity of 180 mA h g–1 with a discharge plateau near 2.0 V (vs Ca2+/Ca) at 100 mA g–1 using an electrolyte based on the salt calcium tetrakis(hexafluoroisopropyloxy)borate (Ca(B(hfip)4)2) in 1,2-dimethoxyethane (DME) and Ca metal. The reversible electrochemical reaction between calcium and selenium is investigated using operando synchrotron-based techniques and the possible reaction mechanism discussed.