Abstract
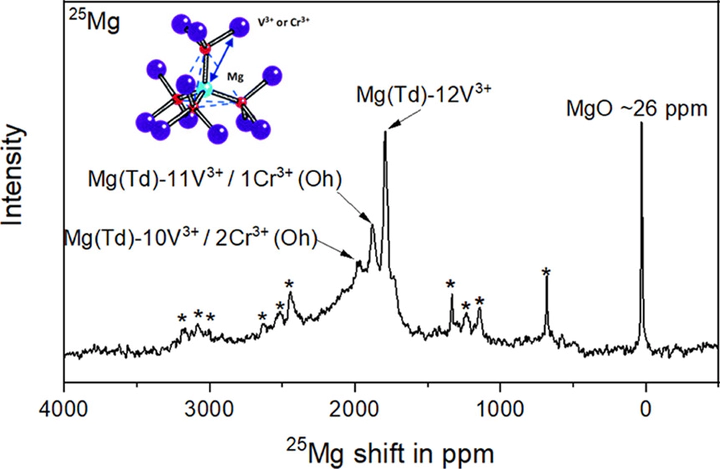
Mg migration in oxide spinels is impeded by strong affinity between divalent Mg and oxygen, suggesting a necessity of exploring new chemistry of solid lattices for functional Mg-ion electrode materials. Cationic mobility with a suitable activation energy in Cr spinels is evidenced by theoretical and experimental results, while redox potentials of V are appropriate to operate with currently limited candidates of nonaqueous electrolytes. By controlling the structure, composition, and complexity, a largely solid-solution MgCrVO4 spinel was synthesized, which, unlike nanocomposites, can bring together the advantages of each transition metal in the lattice. The spinel was successfully synthesized by a simple solid-state reaction with minor inactive Cr- or V-rich components, which was confirmed via 25Mg MAS NMR and high-resolution X-ray diffraction analyses. A thermally and anodically stable Mg(TPFA)2/triglyme electrolyte was utilized for high-temperature electrochemistry and lowering kinetic barriers at and across interfaces so as to observe intercalation behavior of Mg in the designed lattice. Multimodal characterization confirmed an apparent bulk demagnesiation from MgCrVO4 with partial reversibility by probing evolution of the local and long-range structure as well as vanadium and chromium electronic states within the lattice. Characterization experiments also provided a direct evidence for (de)intercalation reactions that occurred without any major competitive conversion reactions or insertion of protons into the lattice, except for the formation of a surface rock salt phase upon charge. These findings in Mg-ion activity expand opportunities to design Mg spinel oxide materials while highlighting the need to identify the origins of reversibility challenges due to, but not limited to, phase stability, particularly for the charged states, barriers at the interface, electrolyte stabilities, and desolvation phenomena, collectively hindering practical use as cathode materials.