Abstract
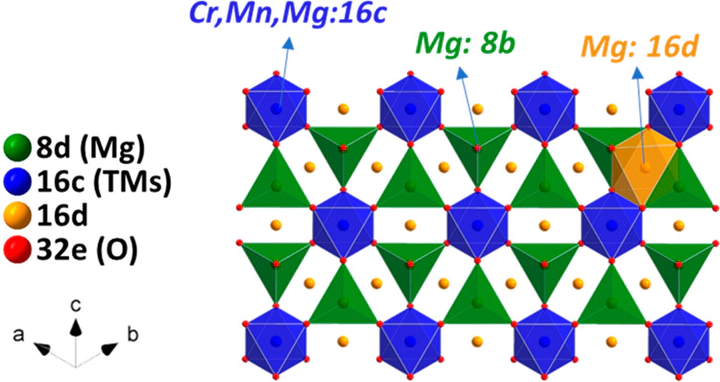
Lattice Mg2+ in a tailored solid solution spinel, MgCrMnO4, is electrochemically utilized at high Mn-redox potentials in a nonaqueous electrolyte. Complementary evidence from experimental and theoretical analyses supports bulk Mg2+ (de)intercalation throughout the designed oxide frame where strong electrostatic interaction between Mg2+ and O2– exists. Mg/Mn antisite inversion in the spinel is lowered to ∼10% via postannealing at 350 °C to further improve Mg2+ mobility. Spinel lattice is preserved upon removal of Mg2+ without any phase transformations, denoting structural stability at the charged state at a high potential ∼3.0 V (vs Mg/Mg2+). Clear remagnesiation upon first discharge, harvesting up to ∼180 Wh/kg at 60 °C is shown. In the remagnesiated state, insertion of Mg2+ into interstitial sites in the spinel is detected, possibly resulting in partial reversibility which needs to be addressed for structural stability. The observations constitute a first clear path to the development of a practical high voltage Mg-ion cathode using a spinel oxide.