The effect of water vapor on surface oxygen exchange kinetics of thin film (La,Sr)(Co,Fe)O3-δ
Abstract
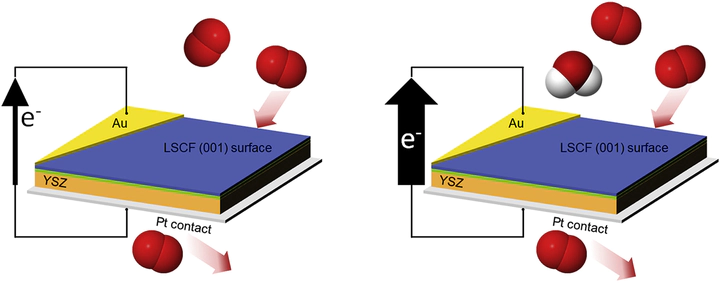
Oxygen reduction and transport in electrochemical applications are strongly affected by the reactant gas composition, including impurities such as water. Understanding the relationship between oxygen reactivity and humidity is key for attaining stability and high efficiency of solid oxide fuel cells, which are especially attractive for transportation applications as low emission power generation devices. Both short- and long-term effects of moisture have previously been associated with use of ambient air. To better understand these effects, we study oxygen exchange kinetics at the cathode surface and the cathode/electrolyte interface using La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) epitaxial thin films as a model-surface system. In situ synchrotron X-ray techniques evaluate the oxygen reduction reaction (ORR) kinetics in response to environmental variables. The results suggest a clear enhancement of the ORR rate upon short term exposure of the perovskite-structured films to water. On the basis of these measurements, along with in situ X-ray characterization of Sr segregation and computational first-principles studies, we suggest a model for this increased ORR activity that may lead to further improvements by stabilizing active cathode surface configurations.