Thermodynamic and kinetic properties of layered-CaCo2O4 for the Ca-ion batteries: a systematic first-principles study
Abstract
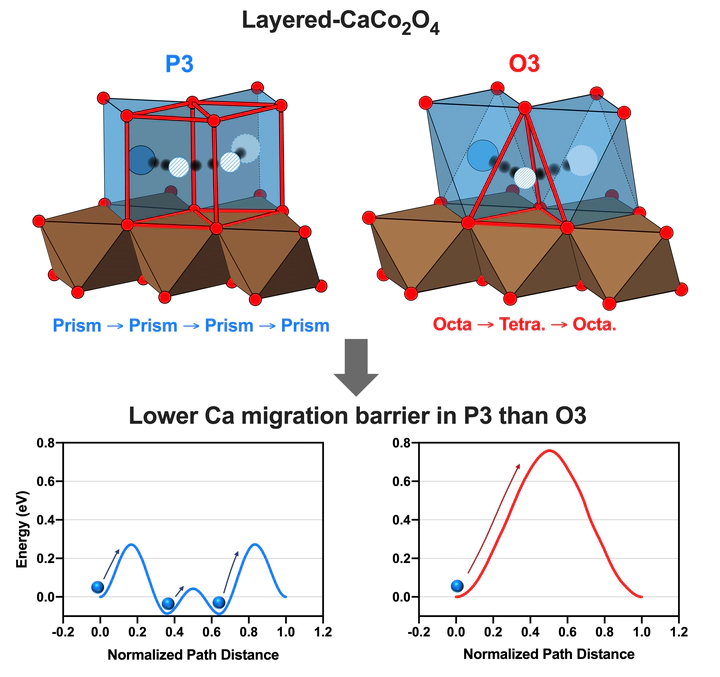
One of the more promising directions in multivalent energy storage is systems based on Ca ion intercalation due to the potential for high voltage and capacity. A major challenge for enabling such a battery is to find cathode materials capable of fast ionic diffusion and reversible insertion of Ca ions. Here, on the basis of first-principles calculations, we have demonstrated that layered CaCo2O4 exhibits favorable thermodynamic and kinetic properties that should enable topotactic Ca ion intercalation reactions. The P3-type layered CaxCo2O4 (0 < x < 1) with either of space groups of P1 or P21/m are stable at multiple Ca concentrations and show a smooth voltage plateau higher than 3 V up to X = 0.5. The energy barriers of the single Ca ion migration are as low as 0.36 eV and 0.27 eV at the dilute and high vacancy concentration limits, respectively. Although varying the vacancy environments of the diffusing atom influences the migration barriers, they do not exceed 0.6 eV. Stochastic analysis of Ca hopping events performed by ab initio molecular dynamics (AIMD) simulation has shown that the migration barriers are lower than 0.32 eV. Therefore, the Ca diffusivity at room temperature extrapolated from the AIMD results is comparable to Li diffusivity (>10−10 cm2s−1) in conventional Li cathode materials, suggesting the feasibility of layered CaxCo2O4 as multivalent cathode materials. Finally, the structural factors that enable fast diffusion are discussed.