Tuning the Adsorption of Polysulfides in Lithium–Sulfur Batteries with Metal–Organic Frameworks
Abstract
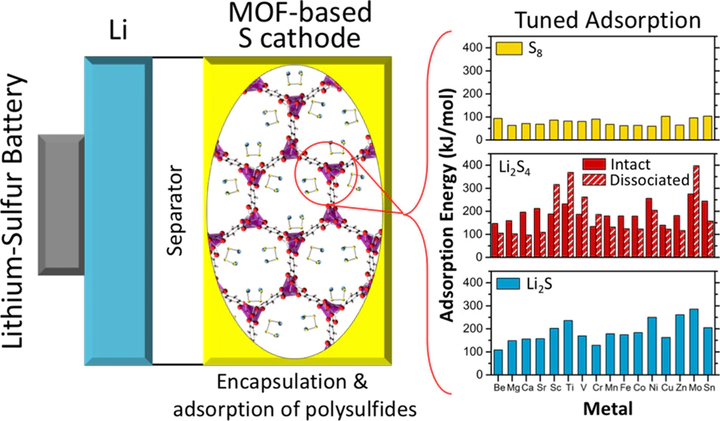
The dissolution of polysulfide (PS) intermediates during discharge is a well-known obstacle to achieving long cycle life in lithium–sulfur batteries. Prior work has shown that PS dissolution can be partially suppressed via physical encapsulation of sulfur and PS within a porous cathode support. Metal–organic frameworks (MOFs) are crystalline, nanoporous materials with extremely high surface areas, whose structure and composition can be varied extensively. MOFs are promising cathode support materials because the encapsulation afforded by MOF pores can be augmented by chemical adsorption of PS onto coordinately unsaturated metal sites (CUS). Here, we demonstrate that this additive approach—restricting PS dissolution by combining encapsulation and adsorption within a MOF—can be tuned to maximize PS anchoring via metal substitution on the CUS. Optimal MOF compositions are pinpointed by computationally screening 16 metal-substituted variants of M2(dobdc) (MOF-74) for their ability to chemically anchor prototypical species (S8, Li2S4, and Li2S) present during the cycling of Li–S batteries. Ti2, Ni2, and Mo2(dobdc) are identified as the compositions with the largest affinities for Li2S4 and Li2S. As Ni2(dobdc) has been synthesized previously, this MOF is proposed as a promising cathode support for Li–S batteries.